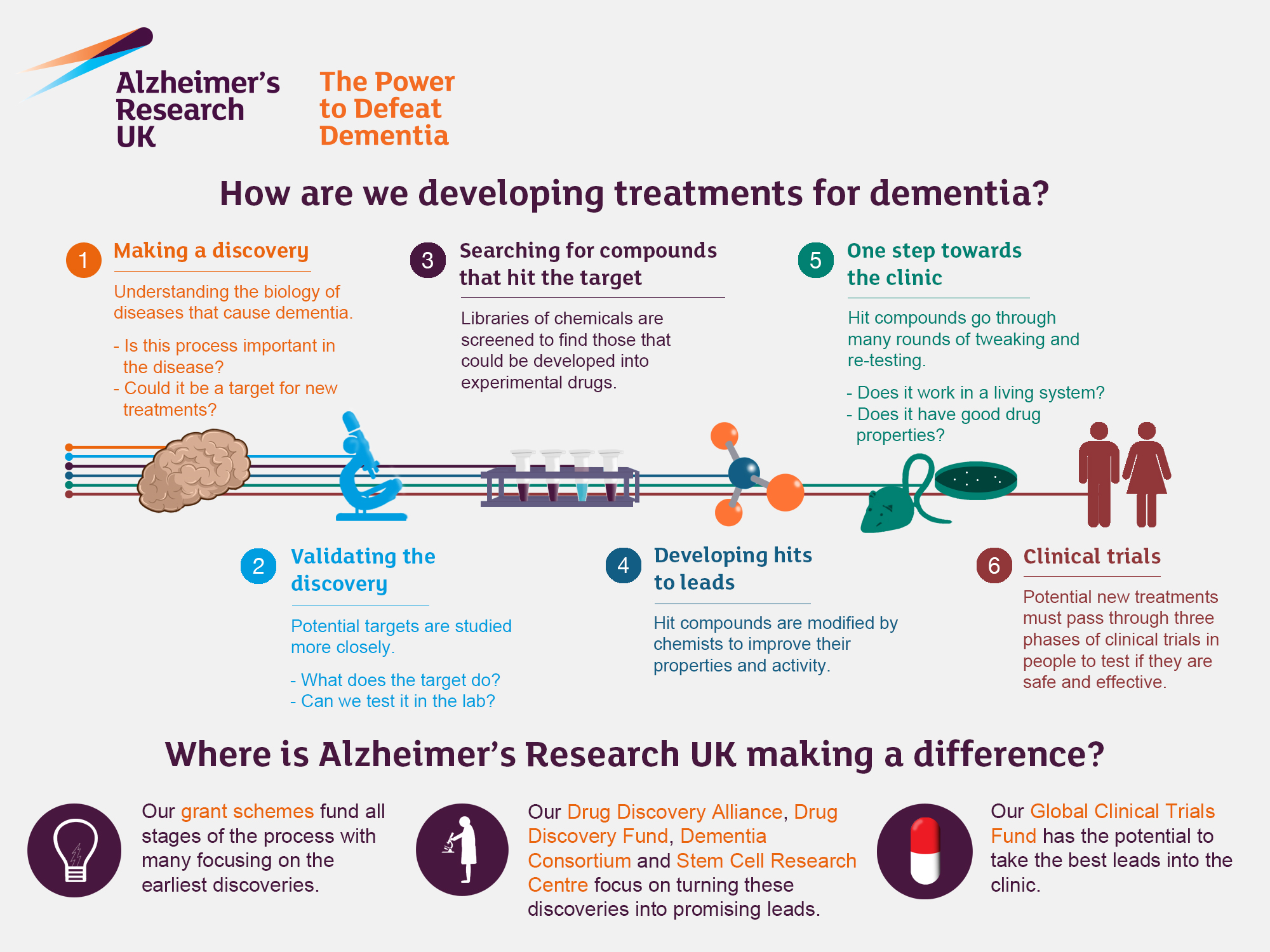
Alzheimer’s research is gaining momentum as scientists strive to unravel the complexities of Alzheimer’s disease, a growing concern for millions worldwide. In particular, the role of microglial cells, the brain’s immune system, is becoming increasingly significant in understanding this neurodegenerative disease. Led by experts like Beth Stevens, groundbreaking studies are revealing how these cells fail to function properly, potentially leading to the devastating effects of Alzheimer’s. By examining the processes involved in immune responses, researchers are developing new treatments aimed at stopping or even reversing the progression of these disorders. As the population ages and Alzheimer’s disease cases are forecasted to surge, the implications of this research could reshape the future of healthcare and treatment options for millions of affected individuals.
Investigating cognitive decline is critical as the prevalence of Alzheimer’s disease and similar neurodegenerative disorders continues to rise. Researchers are focusing on the dynamic relationships between brain cells, particularly the immune-like microglia that help maintain neural health. Prominent figures, such as Beth Stevens, are at the forefront of this inquiry, seeking innovative solutions for managing cognitive impairments. By delving deeper into the biological mechanisms behind these conditions, scientists hope to uncover new medicines and therapies that may significantly alter patient outcomes. As awareness of these issues grows, the urgency to address Alzheimer’s through dedicated research initiatives becomes increasingly paramount.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells are a crucial component of the brain’s immune system, playing a vital role in maintaining neural health and function. As our understanding of Alzheimer’s disease advances, the significance of these cells has become increasingly apparent. According to Beth Stevens, a leading researcher in this field, microglia not only defend against disease but also actively participate in synaptic pruning, a process essential for proper neuronal communication. However, aberrant pruning has been linked to the progression of Alzheimer’s disease and other neurodegenerative diseases, indicating that these cells can become detrimental when functioning incorrectly.
Research conducted at the Stevens Lab has shown that when microglial cells mistakenly clear healthy neurons instead of damaged ones, it leads to synaptic loss associated with cognitive decline in Alzheimer’s patients. This finding underscores the need for further study into the mechanics of microglial activity and their role in Alzheimer’s pathology. By targeting these immune cells, researchers hope to unveil new treatments that can mitigate the effects of neurodegeneration and possibly restore cognitive function, thereby enhancing the quality of life for millions affected by Alzheimer’s.
The Impact of Federal Funding in Alzheimer’s Research
Federal funding plays an instrumental role in advancing Alzheimer’s research, providing essential resources for groundbreaking discoveries in the field. As Beth Stevens shares, support from the National Institutes of Health and various federal agencies has been pivotal in exploring the complexities of microglial cells and their implications for Alzheimer’s disease. These financial investments allow researchers to delve into basic science, which inspires innovative hypotheses and potential treatments for neurodegenerative diseases.
Without such foundational funding, many significant advancements in understanding the nuances of Alzheimer’s disease may never come to fruition. The successful translation of early-stage findings into practical applications hinges on sustained financial backing. As the U.S. faces a growing aging population, the importance of continued investment in Alzheimer’s research cannot be overstated. Effective allocation of resources to studies focused on microglia and other neuroimmune mechanisms promises not only to uncover new biomarkers for early detection but also to develop treatments that could alter the disease’s trajectory.
Revolutionizing Treatments for Neurodegenerative Diseases
The quest for new treatments in Alzheimer’s and related neurodegenerative diseases requires innovative approaches and fresh perspectives. Research led by Beth Stevens has opened new avenues for understanding how neuroimmune interactions can influence disease outcomes. By focusing on microglial cells, Stevens’s work highlights the potential of targeting immune pathways as a promising treatment strategy. This paradigm shift in thinking about Alzheimer’s could lead the way to therapies that restore synaptic health and improve cognitive function in patients.
Furthermore, as the landscape of Alzheimer’s research progresses, interdisciplinary collaborations are becoming increasingly vital. Merging insights from neuroscience, immunology, and genetics allows scientists to tackle the disease from multiple angles, potentially resulting in more effective interventions. With the projected rise in Alzheimer’s cases, the urgency for groundbreaking therapeutics has never been more pressing. The insights gained from Stevens’s research may serve as the foundation for future innovations that could redefine the therapeutic landscape for neurodegenerative diseases beyond just Alzheimer’s.
The Role of Curiosity-Driven Science in Neuroimmunology
Curiosity-driven science is a fundamental aspect of research that leads to unexpected breakthroughs, particularly in fields like neuroimmunology. Beth Stevens’s experience illustrates how following scientific inquiry can unveil insights into brain functionality and its associated diseases. While some critics may question the relevance of studying basic biological mechanisms, Stevens’s findings about microglial cells highlight the intricate connections between immune response and cognitive health, illustrating the importance of foundational research in paving the way for future therapies.
Moreover, the journey of discovery often involves grappling with uncertainty and exploring seemingly unrelated phenomena, which can eventually yield significant implications for disease treatment. By venturing into less-traveled paths of investigation, researchers can glean insights that challenge conventional wisdom and lead to novel therapeutic avenues in Alzheimer’s disease and other neurodegenerative conditions. This underscores the critical need for continued support and funding for exploratory research that fuels innovation in the fight against Alzheimer’s.
Beth Stevens: A Pioneer in Alzheimer’s Research
Beth Stevens stands out as a pioneer in Alzheimer’s research, shifting the narrative surrounding microglial cells and highlighting their crucial role in the brain’s immune system. Her groundbreaking work has not only enhanced our understanding of how these cells contribute to neurodegenerative diseases but has also laid the groundwork for developing innovative treatment strategies. Recognized for her excellence with accolades like the MacArthur Fellowship, Stevens exemplifies how dedication to scientific inquiry can lead to significant advancements in understanding and potentially combating Alzheimer’s.
Through her research at Boston Children’s Hospital and the Broad Institute, Stevens has made pivotal contributions to uncovering how immune processes in the brain can influence neuronal health and synaptic function. Her laboratory has become a hub for studying the underlying mechanisms of diseases like Alzheimer’s, paving the way for the development of new medicines and early biomarkers that could revolutionize patient care and intervention strategies.
The Importance of Early Detection in Alzheimer’s Disease
Early detection of Alzheimer’s disease is critical for effective intervention and management of this neurodegenerative disorder. As research advances, understanding the biological markers and cognitive signs of Alzheimer’s is becoming increasingly crucial. The work of researchers like Beth Stevens emphasizes the need to identify changes in microglial behavior that could serve as precursors to clinical symptoms. By recognizing these changes early, it may be possible to implement strategies that slow the progression of the disease.
Moreover, developing reliable biomarkers for early detection holds the promise of not only improving patient outcomes but also reducing the economic burden associated with Alzheimer’s care. With the anticipated rise in cases due to an aging population, investing in research that focuses on early diagnosis and effective treatment pathways is essential for the future of Alzheimer’s care. By prioritizing early intervention, healthcare systems may significantly alter the trajectory of Alzheimer’s for millions.
Understanding Neurodegenerative Disease Mechanisms
The mechanisms underlying neurodegenerative diseases, including Alzheimer’s, are complex and multifaceted. Research has shown that interactions between neuroinflammation and neurodegeneration are critical in understanding how these diseases progress. Microglial cells, as highlighted in Beth Stevens’s work, play an essential role in these processes, and investigating their behavior can provide insights into the early and late stages of neurodegenerative diseases. This knowledge is vital for the development of targeted therapies aimed at modulating immune responses in the brain.
Furthermore, a comprehensive understanding of the interconnected pathways involved in Alzheimer’s is necessary to develop effective treatment options. By exploring the roles of various cell types, including microglia, and their interactions within the neural environment, researchers can identify new therapeutic targets. This approach not only aims to halt the progression of Alzheimer’s disease but also seeks to restore cognitive function in affected individuals, marking a significant shift in treatment paradigms.
Future Directions in Alzheimer’s Treatment Research
The future of Alzheimer’s treatment research is being shaped by innovative studies that focus on the roles of various cellular mechanisms, particularly concerning microglial function. Current investigations aim to assess how modulating the activity of these immune cells can lead to therapeutic breakthroughs in Alzheimer’s disease. By capitalizing on the insights gained from basic research, scientists hope to develop novel pharmacological interventions that directly target the dysfunctional aspects of microglia in Alzheimer’s patients.
In addition to pharmacological approaches, emerging technologies such as gene editing and immunotherapy are being explored as potential therapies for Alzheimer’s disease. By harnessing advancements in medical science, researchers remain optimistic about finding new avenues for treatment that not only address symptoms but also target the underlying causes of neurodegeneration. As the field continues to evolve, the hope is that these innovative approaches will lead to significant improvements in the quality of life for individuals living with Alzheimer’s.
Collaboration in Alzheimer’s Research: A Pathway to Success
Collaboration is a cornerstone of successful Alzheimer’s research, bringing together diverse expertise to tackle the complex challenges posed by this neurodegenerative disease. Researchers like Beth Stevens advocate for partnerships between institutions, bringing together neuroscientists, immunologists, and geneticists to create a comprehensive understanding of Alzheimer’s pathology. These collaborative efforts foster innovation and enable researchers to share resources, ideas, and findings that can accelerate the pace of discovery and treatment.
Additionally, collaboration extends beyond academia to include partnerships with biotech companies and healthcare organizations. This synergy helps translate research findings into clinical applications more effectively. With a shared mission to combat Alzheimer’s disease, these collaborations can streamline the development of new therapies, from bench to bedside, ensuring that scientific advancements result in tangible benefits for patients facing the challenges of dementia.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are critical in Alzheimer’s research as they serve as the brain’s immune system. They patrol for signs of illness and injury, clearing dead or damaged cells and pruning synapses. Findings from researchers like Beth Stevens indicate that abnormal microglial activity can contribute to the progression of Alzheimer’s disease, leading to new insights and potential treatments.
How is Beth Stevens contributing to the understanding of Alzheimer’s disease?
Beth Stevens is significantly advancing Alzheimer’s research by exploring the functions of microglial cells. Her work reveals how these immune cells affect synapse pruning, which, when disrupted, can lead to neurodegenerative diseases like Alzheimer’s. Her findings are paving the way for new treatments and biomarkers for early diagnosis.
What breakthroughs have been achieved in neurodegenerative diseases through Alzheimer’s research?
Recent breakthroughs in Alzheimer’s research focus on understanding the role of microglial cells in neurodegenerative diseases. Studies by Beth Stevens demonstrate that improper pruning by microglia can exacerbate conditions such as Alzheimer’s disease, leading to innovative approaches for new treatments aiming to correct these processes.
Why are new treatments for Alzheimer’s disease considered essential?
New treatments for Alzheimer’s disease are essential due to the rising number of cases, projected to double by 2050. Increasing research efforts, especially those highlighting microglial functions, are vital for developing effective therapies that could improve the quality of life for millions affected by Alzheimer’s and related neurodegenerative diseases.
How does the research on microglial cells influence potential new therapies for Alzheimer’s?
Research on microglial cells is influential in developing new Alzheimer’s therapies by revealing their role in synaptic health. Abnormal microglial behavior has been linked to Alzheimer’s progression, and understanding these dynamics allows scientists to target new treatment strategies aimed at restoring proper microglial functions, potentially altering disease outcomes.
What future directions are being explored in Alzheimer’s research?
Future directions in Alzheimer’s research include identifying biomarkers through the study of microglial cells and understanding their pivotal role in neuroinflammation. Leaders like Beth Stevens are focusing on how these insights can lead to early diagnosis and the development of innovative therapies that may halt or reverse Alzheimer’s disease progression.
What impact does federal funding have on Alzheimer’s research?
Federal funding plays a crucial role in Alzheimer’s research, supporting foundational studies that explore brain mechanisms and cellular responses. Researchers like Beth Stevens attribute early career successes and breakthroughs in understanding microglial cells and Alzheimer’s disease to the essential backing provided by the National Institutes of Health and other federal agencies.
| Key Point | Details |
|---|---|
| Microglial Cells | Shrinkage of microglia is involved in disease processes like Alzheimer’s. |
| Role in the Brain | They clear out dead or damaged cells and prune synapses, affecting neuron communication. |
| Aberrant Pruning | Dysfunctional pruning may lead to neurodegenerative diseases. |
| Research Impact | Stevens’ work has attracted federal funding and aims at developing new treatments. |
| Funding Sources | Major support from the National Institutes of Health. |
| Future Predictions | By 2050, Alzheimer’s cases expected to double, raising care costs significantly. |
| Research Foundations | Basic, curiosity-driven science is crucial for real-world disease applications. |
Summary
Alzheimer’s research is making significant strides with new discoveries related to microglial cells, which play a pivotal role in brain health. The work of researchers like Beth Stevens highlights the importance of fundamental science in understanding complex diseases. By investigating how microglia function and their role in synaptic pruning, we can uncover new therapies for the millions affected by Alzheimer’s. As the aging population grows, sustainable research efforts are essential to address the impending increase in cases and healthcare costs related to this devastating disease.