Research on TIM-3 Alzheimer’s treatment is opening new doors in the fight against Alzheimer’s disease, showing promise in improving cognitive function and amyloid beta clearance in affected patients. Recent findings highlight how inhibiting TIM-3, an immune checkpoint molecule, can unleash microglia—the brain’s immune cells—to effectively clear harmful plaques associated with Alzheimer’s. This innovative approach mirrors successful strategies used in cancer therapies, where checkpoint inhibitors restore immune function. By targeting TIM-3, researchers aim to reverse cognitive decline, presenting a beacon of hope amid the ongoing struggles in Alzheimer’s drug trials. As our understanding deepens, TIM-3 may well become a cornerstone in the effort to combat this debilitating condition, reshaping treatment landscapes with its thoughtful engagement of the immune system.
The emergence of TIM-3 as a potential therapeutic target has sparked interest in rethinking how we approach Alzheimer’s therapy. This novel treatment strategy leverages immune checkpoint inhibitors to enhance the role of microglia, which are crucial in maintaining brain health by clearing amyloid beta and related plaques. By modifying the pathways that regulate these immune cells, researchers believe it is possible to foster cognitive improvement and potentially slow the progression of Alzheimer’s disease. In this context, therapies aimed at TIM-3 could pave the way for breakthroughs in managing late-onset Alzheimer’s, marking a significant shift in therapeutic paradigms. As we explore these developments, the landscape of Alzheimer’s treatment continues to evolve, holding promise for better patient outcomes.
Understanding TIM-3’s Role in Alzheimer’s Disease
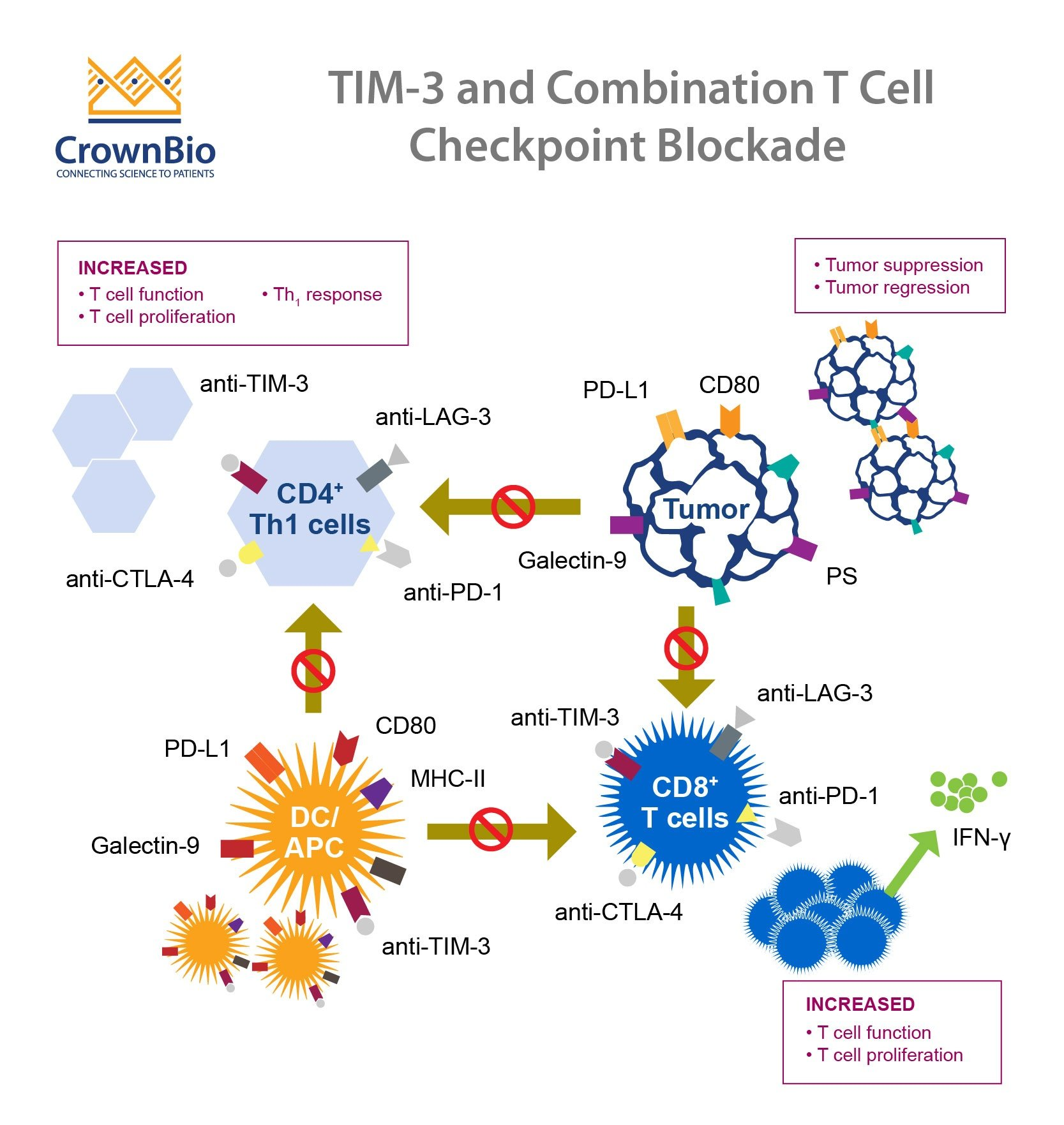
TIM-3, or T-cell immunoglobulin mucin 3, is an immune checkpoint molecule that has gained attention for its dual role in both cancer immunotherapy and Alzheimer’s disease. In the context of Alzheimer’s, TIM-3 inhibits the activity of microglia, the brain’s immune cells. These cells play a crucial role in clearing harmful plaques, such as amyloid beta, that accumulate in the brains of Alzheimer’s patients. Research indicates that microglia in individuals with Alzheimer’s show heightened levels of TIM-3 expression, leading to diminished plaque clearance and exacerbating cognitive decline.
The innovative aspect of recent studies is the exploration of TIM-3 deletion as a therapeutic strategy. Mice genetically modified to lack TIM-3 exhibited improved cognitive function and enhanced clearance of amyloid plaques. This compelling evidence suggests that targeting TIM-3 may release the brakes on microglial activity, allowing these cells to fulfill their role in maintaining brain health, highlighting the potential for TIM-3-related therapies in the fight against Alzheimer’s.
The Mechanism Behind Microglial Dysfunction in Alzheimer’s Disease
Microglia are the primary immune cells in the brain, responsible for maintaining homeostasis and responding to injury or disease. In Alzheimer’s disease, however, the normal function of microglia is disrupted due to the overexpression of negative regulators like TIM-3. When activated by Alzheimer’s-related pathology, these microglial cells increasingly express TIM-3, which effectively dampens their ability to engulf amyloid beta and other neurotoxic materials. This impairment leads to a failure in the clearance of toxic plaques, further contributing to neuronal damage and cognitive decline.
Understanding the signaling pathways involved in TIM-3 expression is crucial for developing effective interventions. Current strategies may focus on inhibiting TIM-3 to rejuvenate microglial activity, encouraging them to clear amyloid deposits. By restoring the balance between activation and inhibition, researchers hope to improve cognitive outcomes, highlighting the importance of microglial health in Alzheimer’s pathology.
Potential of Immune Checkpoint Inhibitors in Alzheimer’s Treatment
The concept of utilizing immune checkpoint inhibitors—molecules like TIM-3 that can modulate immune responses—opens a novel avenue in Alzheimer’s treatment. These therapies, already being employed for certain cancers, may potentially increase the efficacy of microglial clearance of amyloid beta in Alzheimer’s patients. By blocking TIM-3, we can potentially unleash the brain’s immune defenses, enabling microglia to more effectively combat the plaque buildup that is characteristic of the disease.
This approach represents a shift in thinking about Alzheimer’s therapy, moving towards strategies that enhance the patient’s own immune response rather than relying solely on conventional amyloid-targeted drugs. The preliminary studies using TIM-3 inhibitors in animal models have shown promising results in improving cognitive function, prompting further exploration and paving the way for new Alzheimer’s drug trials focused on immune modulation.
The Link Between TIM-3 and Amyloid Beta Clearance
In Alzheimer’s disease, the accumulation of amyloid beta plaques in the brain is a defining hallmark. Studies suggest that the overexpression of TIM-3 on microglia hampers their ability to clear these plaques, leading to progressive neurodegeneration. When TIM-3’s inhibitory function is disrupted, microglia can surge into action, engulfing the amyloid beta and facilitating its clearance from the brain, which is essential for maintaining cognitive function and overall brain health.
This relationship between TIM-3 and amyloid beta underscores a critical target for therapeutic intervention. Targeting TIM-3 could serve as a strategy for enhancing amyloid clearance, potentially reversing some of the cognitive deficits observed in Alzheimer’s models. Consequently, TIM-3 antagonists represent a promising avenue in therapeutic development, aiming for cognitive improvement in individuals suffering from Alzheimer’s disease.
Cognitive Improvement through TIM-3 Modulation
Recent studies have illustrated that modifying TIM-3 expression can lead to significant cognitive improvement in Alzheimer’s mouse models, suggesting that therapeutic strategies targeting this immune checkpoint may yield restorative effects on cognition. By freeing microglia to effectively clear amyloid plaques and restore synaptic function, researchers have noted marked enhancements in memory and learning abilities in treated mice. This improvement is characterized by their regained ability to navigate mazes and reduced anxiety levels, indicating a potential restoration of normal cognitive functions.
In light of these findings, TIM-3 modulation offers a novel treatment strategy that not only aims to clean the brain of amyloid beta but also seeks to restore cognitive capabilities in patients with Alzheimer’s disease. By harnessing the immune system’s potential, we may finally find effective interventions for a condition that has long resisted treatment breakthroughs.
Alzheimer’s Drug Trials: The Future of TIM-3 Therapeutics
The pathway towards clinical application of TIM-3 therapies in Alzheimer’s disease is marked by ongoing drug trials aimed at evaluating the safety and efficacy of anti-TIM-3 agents. Given the historical challenges faced in Alzheimer’s drug testing, the prospect of leveraging immune checkpoint inhibitors offers a refreshing strategy that diverges from traditional approaches focused solely on amyloid targets. Drug trials are increasingly integrating checkpoints as novel modalities to potentiate the effects of existing treatments.
As research continues to unravel the potential of TIM-3 modulation, these trials stand at the forefront of innovation in Alzheimer’s treatment. The hope is that successful outcomes will lead to new FDA-approved therapies, providing patients with more effective options for combating cognitive decline associated with Alzheimer’s disease.
The Role of Microglia in Alzheimer’s Pathology
Microglia, the resident immune cells of the brain, play a pivotal role in both the development and pathology of Alzheimer’s disease. They are responsible for surveillance and maintaining homeostasis in the central nervous system. However, in the presence of amyloid beta, the microglial response is often inadequate due to the upregulation of inhibitory checkpoint molecules like TIM-3, which dampens their ability to act effectively.
In Alzheimer’s pathology, when microglia are unable to clear amyloid plaques, a vicious cycle ensues. The continued accumulation of these plaques leads to chronic inflammation and neurodegeneration, exacerbating cognitive decline. Understanding how to enhance microglial function and reverse this impairment represents a critical area of focus in developing future Alzheimer’s treatment strategies.
Implications of TIM-3 Research for Future Therapies
The research focused on TIM-3 in the context of Alzheimer’s disease has significant implications for future therapeutic strategies. By understanding the mechanisms that prevent microglial clearance of amyloid beta, researchers are paving the way for a new generation of treatments that could significantly alter the trajectory of the disease. The potential to repurpose existing TIM-3 antibodies used in cancer treatment for Alzheimer’s highlights an exciting opportunity to expedite the development of effective therapies.
Furthermore, as ongoing studies aim to investigate the translational potential of TIM-3 inhibition in human models, the scientific community will be closely monitoring outcomes. If successful, this approach could mark a pivotal shift in how we conceptualize and treat Alzheimer’s disease, focusing more on immune modulation as a key therapeutic avenue.
The Future of Alzheimer’s Research and Immunotherapy
Research into Alzheimer’s disease is evolving, with an increasing emphasis on immunotherapy strategies, including the exploration of TIM-3. By integrating findings from immunology and neurology, scientists are creating a more comprehensive understanding of how immune mechanisms influence Alzheimer’s pathology. The implications for treatment are profound, as this approach aims to harness the body’s immune system to combat neurodegeneration.
As new clinical trials emerge and innovative therapies targeting TIM-3 are developed, the landscape of Alzheimer’s treatment is poised for change. This integration of immunotherapy presents not only a promising direction for drug development but also a hopeful future for patients and families affected by this debilitating disease.
Frequently Asked Questions
What role do immune checkpoint inhibitors play in TIM-3 Alzheimer’s treatment?
Immune checkpoint inhibitors, like TIM-3, serve to modulate the immune response in Alzheimer’s treatment. By blocking the TIM-3 molecule, microglia—brain immune cells—are released from inhibition, allowing them to effectively clear amyloid plaques that contribute to Alzheimer’s disease and improve cognitive function.
How does TIM-3 influence microglia in Alzheimer’s treatment?
In the context of Alzheimer’s treatment, TIM-3 negatively regulates microglial activity. By inhibiting TIM-3, microglia can more effectively engage in the clearance of amyloid beta plaques, thus potentially restoring cognitive function and alleviating memory deficits.
Can TIM-3 Alzheimer’s treatment lead to cognitive improvement?
Yes, TIM-3 Alzheimer’s treatment has shown promising results in enhancing cognitive improvement in lab models. By deleting the TIM-3 gene, researchers observed improved memory and cognitive behavior in mice, indicating that targeting TIM-3 may offer a therapeutic pathway for Alzheimer’s patients.
What are the potential benefits of targeting TIM-3 in Alzheimer’s drug trials?
Targeting TIM-3 in Alzheimer’s drug trials has the potential to bring forth significant benefits, including enhanced clearance of neurotoxic amyloid plaques and improved cognitive function. This novel approach could provide an alternative to traditional Alzheimer’s drugs, which have limited effectiveness.
How does TIM-3 impact amyloid beta clearance in Alzheimer’s?
TIM-3 inhibits microglial phagocytic activity, impeding the clearance of amyloid beta. By blocking TIM-3, researchers aim to enhance the capability of microglia to remove these harmful plaques from the brain, which could lead to improved outcomes in Alzheimer’s treatment.
| Key Points |
|---|
| A study highlights TIM-3 as a target for Alzheimer’s treatment, similar to cancer therapies. |
| Deleting TIM-3 allows microglia to better clear amyloid plaques and improves memory in mice. |
| Approximately 90-95% of Alzheimer’s cases are late-onset, with TIM-3 linked to this type. |
| Microglia, the brain’s immune cells, are hindered by TIM-3 from attacking harmful plaques. |
| Research indicates that anti-TIM-3 antibodies could be repurposed for Alzheimer’s treatment. |
| Study conducted over five years; collaboration between multiple researchers and institutions. |
| Future research aims to test human anti-TIM-3 in Alzheimer’s mouse models. |
Summary
TIM-3 Alzheimer’s treatment represents a promising breakthrough in the fight against this devastating disease. A new study demonstrates that targeting TIM-3, an immune checkpoint molecule, can significantly enhance the brain’s ability to clear harmful amyloid plaques and improve memory function in mice. This innovative approach, which has drawn inspiration from cancer therapies, highlights a potential pathway to address one of the most critical barriers in Alzheimer’s treatment: the ineffective clearance of accumulated plaques by microglia. As researchers work towards developing effective human therapies based on this mechanism, the prospects for patients suffering from Alzheimer’s are becoming increasingly optimistic.