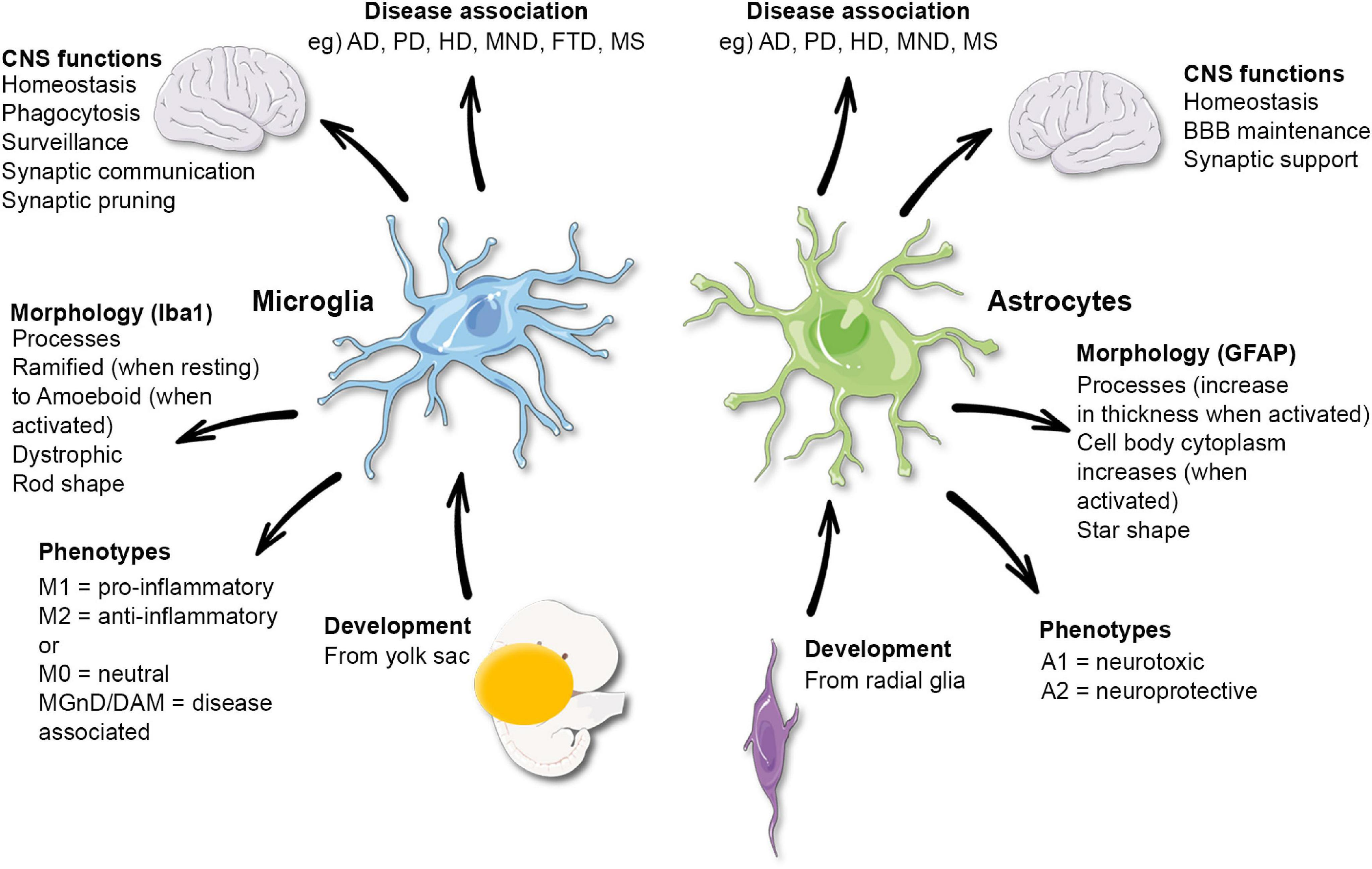
Microglial research is transforming our understanding of the brain’s immune system and its role in neurological disorders. These specialized cells not only protect the brain by clearing out dead or damaged neurons, but they also play a crucial part in synaptic pruning, refining the connections between nerve cells. However, aberrant microglial functioning has been linked to neurodegenerative diseases such as Alzheimer’s disease and Huntington’s disease, complicating their protective roles. This burgeoning field of study has significant implications for developing new biomarkers and therapies aimed at treating the millions impacted by Alzheimer’s, a disease currently lacking a cure. As scientists delve deeper into microglial behaviors, they unravel the complex relationships between immune responses and brain health, paving the way for innovative interventions against debilitating conditions.
Investigations into the roles of glial cells, especially microglia, are redefining paradigms in neuroscience and illuminating pathways that affect various brain functions. By acting as the brain’s surveillance team, these cells are essential for maintaining neurological integrity and ensuring that neural networks develop correctly through processes such as synaptic refinement. Yet, when these immune cells misbehave, they may contribute to the progression of diseases like Alzheimer’s and other neurodegenerative conditions. This research holds promise for discovering novel therapeutic measures to combat cognitive decline, especially as current treatments for Alzheimer’s remain inadequate. As we continue to explore microglial functionalities, we not only enhance our comprehension of brain biology but also aim to mitigate the impact of serious health challenges.
The Role of Microglial Research in Understanding Alzheimer’s Disease
Microglial research has become a critical component in understanding the complexities of Alzheimer’s disease. These specialized immune cells are fundamental to the brain’s defense system, responding to injury and inflammation by clearing away debris and damaged cells. However, in the context of Alzheimer’s, dysregulation of microglial activity can lead to excessive synaptic pruning, which is thought to contribute to the cognitive decline observed in patients. By investigating how microglia interact with neurons, researchers hope to identify novel therapeutic targets that can restore healthy brain function.
Beth Stevens’ pioneering work in microglial research exemplifies this endeavor. By elucidating the mechanisms by which microglia prune synapses, she has opened new avenues for developing biomarkers and potential treatments for Alzheimer’s disease. As millions of Americans grapple with this debilitating condition, the focus on microglial cells signifies a revolutionary shift in how researchers approach neurodegenerative diseases. Enhanced understanding through ongoing studies could eventually lead to breakthroughs that not only slow down the progression of Alzheimer’s but also improve patients’ quality of life.
Unraveling the Brain’s Immune System: Implications for Neurological Disorders
The brain’s immune system, primarily composed of microglial cells, plays a pivotal role in maintaining neurological health. These cells are responsible for surveilling the brain environment, responding to threats, and continuously reshaping neural networks through synaptic pruning. In conditions such as Alzheimer’s, Huntington’s disease, and other neurodegenerative disorders, the delicate balance of microglial activity can become disrupted. Understanding this balance is essential for developing effective interventions that target the underlying mechanisms of these diseases.
In exploring the implications of microglial dysfunction, researchers are making significant strides towards uncovering potential therapeutic strategies. For instance, by identifying how microglia contribute to neuroinflammation and synaptic loss, scientists can devise ways to enhance their protective roles while mitigating harmful effects. In the long term, this could lead to tailored treatments for patients suffering from various neurological disorders, ultimately fostering a more resilient brain immune system and reducing the burden of these debilitating conditions.
The Impact of Synaptic Pruning on Neurodegenerative Diseases
Synaptic pruning, a natural process mediated by microglial cells, is essential for healthy brain development and function. This process allows the brain to eliminate unnecessary synaptic connections, thus refining neural circuitry. However, abnormal synaptic pruning is increasingly recognized as a contributing factor to the pathogenesis of neurodegenerative diseases like Alzheimer’s. When microglial cells become overactive, they can excessively prune synapses, leading to the loss of vital neuronal connections and exacerbating cognitive decline.
Research into synaptic pruning has gained traction in recent years, highlighting the importance of this process in maintaining brain health. Investigators like Beth Stevens are uncovering the intricate balance between protective and harmful microglial functions, paving the way for innovative therapeutic strategies. By harnessing this knowledge, scientists aim to mitigate the detrimental effects of dysregulated synaptic pruning in neurodegenerative diseases, potentially slowing progression and enhancing cognitive function in affected individuals.
Funding Foundations: Supporting Research in Neurodegenerative Diseases
The quest to better understand neurodegenerative diseases has been significantly bolstered by federal funding, particularly from organizations like the National Institutes of Health. This financial support is crucial for pioneering research initiatives that explore the roles of microglial cells and synaptic pruning in disorders like Alzheimer’s disease. As emphasized by Beth Stevens, consistent funding allows scientists to pursue innovative and sometimes unconventional research paths that can ultimately yield transformative insights into brain health.
Sustained investment in fundamental research is essential for translating scientific discoveries into real-world applications. By fostering curiosity-driven exploration, these funding avenues enable researchers to ask critical questions that may lead to breakthroughs in understanding and treating Alzheimer’s and other neurological disorders. Ultimately, the continued support for research in this field is vital for advancing our knowledge of the brain’s immune system and the broader mechanisms that underpin neurodegeneration.
New Biomarkers for Early Detection of Alzheimer’s Disease
The search for reliable biomarkers for Alzheimer’s disease has become a focal point of contemporary research, with significant progress having been made thanks to studies on microglial function. Biomarkers can provide invaluable insights into the early stages of the disease, allowing for earlier diagnosis and intervention. By understanding how microglial cells react in the disease process, researchers are working towards identifying specific markers that signify pathological changes associated with Alzheimer’s.
Beth Stevens’ research has contributed significantly to this area, demonstrating that alterations in microglial activity can serve as critical indicators of disease onset. As the field advances, these biomarkers could enable clinicians to assess disease risk more accurately and tailor prevention strategies for at-risk individuals. Ultimately, improving early detection through biomarker research can lead to timely therapeutic interventions that may slow the progression of Alzheimer’s disease and enhance patient outcomes.
Developing New Therapeutics for Alzheimer’s Disease Management
The quest for effective therapeutics to manage Alzheimer’s disease remains a top priority for researchers globally. The peculiar behavior of microglial cells in brain immunity and their role in synaptic pruning has opened up exciting avenues for drug development. By targeting microglial pathways, scientists are exploring ways to create treatments that not only halt disease progression but also potentially restore cognitive function in patients.
Current therapeutic approaches are limited, highlighting the necessity for continued research into novel compounds that can alter microglial activity. There’s potential for developing agents that can either enhance the beneficial aspects of microglia or inhibit their harmful effects, especially regarding excessive synaptic pruning. The findings from Beth Stevens’ lab underscore the importance of fundamental science in informing these therapeutic endeavors, with the ultimate goal of improving the lives of those affected by Alzheimer’s and related neurodegenerative disorders.
The Importance of Curiosity-Driven Research in Neuroscience
Curiosity-driven research is at the heart of scientific discovery, particularly in the field of neuroscience. Beth Stevens exemplifies this principle through her work on microglial cells and their implications for diseases like Alzheimer’s. By pursuing her curiosity about the brain’s immune system, Stevens has made significant contributions to our understanding of synaptic pruning and its relationship with neurodegeneration. This underscores the vital role of foundational research in revealing complex biological processes that may ultimately lead to practical applications.
Investing in curiosity-driven projects not only fosters innovation but also encourages researchers to explore uncharted territories. As Stevens points out, the unexpected connections and discoveries that arise from such research can have far-reaching implications for our understanding of neurological disorders. It is this spirit of inquiry that drives progress in the field, allowing scientists to develop new hypotheses, refine existing theories, and ultimately enhance treatment strategies for diseases affecting millions worldwide.
Innovations in Imaging Techniques to Study Microglial Activity
Recent advancements in imaging techniques have revolutionized the way researchers study microglial activity within the brain. The ability to visualize these immune cells in real-time has provided unprecedented insights into their role in various neurological disorders, including Alzheimer’s disease. Non-invasive imaging methods are now enabling scientists to track microglial responses to neuronal damage and inflammation, shedding light on their contributions to synaptic pruning and neurodegeneration.
This progress in imaging technology is crucial for developing a deeper understanding of the complexities of the brain’s immune system. By employing sophisticated techniques, researchers can delineate the intricate interactions between microglia and neurons, paving the way for targeted therapeutic interventions. As the field advances, these innovations hold the promise of identifying changes in microglial behavior that could serve as early indicators of neurodegenerative diseases, ultimately leading to improved diagnostic and treatment strategies.
The Future of Alzheimer’s Research: Collaborations and Interdisciplinary Approaches
The future of Alzheimer’s research is increasingly characterized by collaborative and interdisciplinary approaches. Various fields, from molecular biology to neuroimaging, are converging to provide a holistic understanding of the factors driving the disease. As scientists like Beth Stevens emphasize, collaborative efforts facilitate the sharing of knowledge and resources, leading to innovative solutions for combatting neurodegenerative diseases.
Interdisciplinary partnerships also enable researchers to tackle the complexities of diseases like Alzheimer’s from multiple angles, integrating insights about the brain’s immune system with genetic, environmental, and lifestyle factors. This comprehensive approach holds the key to unraveling the multifactorial nature of Alzheimer’s, potentially leading to breakthroughs in treatment and prevention strategies. As the landscape of Alzheimer’s research evolves, fostering collaboration across diverse scientific disciplines will remain paramount in the quest for effective interventions.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial to Alzheimer’s disease research as they function as the brain’s immune system. They monitor neuronal health, clear dead cells, and prune synapses, which can affect neurodegenerative processes. Abnormal microglial activity in Alzheimer’s disease can lead to excessive synaptic pruning, contributing to cognitive decline. Understanding their role offers insights into potential therapeutic targets.
How do microglial cells contribute to neurological disorders beyond Alzheimer’s disease?
Microglial cells are implicated in various neurological disorders beyond Alzheimer’s. Their involvement in neurodegenerative diseases, such as Huntington’s disease, suggests that dysregulation in their function can lead to excessive synaptic pruning and inflammation, exacerbating neuronal loss. Research into microglial mechanisms is essential for developing treatments for multiple neurological disorders.
What is synaptic pruning, and why is it important in microglial research?
Synaptic pruning is the process by which microglial cells eliminate unnecessary synapses in the brain, a crucial mechanism for brain development and function. In microglial research, understanding how this process can become maladaptive in conditions like Alzheimer’s disease is vital. Dysregulated synaptic pruning can lead to disrupted neural circuits and cognitive impairment, highlighting the importance of microglia in maintaining brain health.
Why is it important to study the brain’s immune system in the context of neurodegenerative diseases?
Studying the brain’s immune system, particularly microglial cells, is vital for understanding neurodegenerative diseases. These cells protect against injury and disease, but when dysfunctional, they can contribute to conditions such as Alzheimer’s disease. Insights from microglial research can lead to new biomarkers and therapies, enhancing our ability to diagnose and treat these chronic conditions.
What advancements have been made in microglial research related to Alzheimer’s disease?
Recent advancements in microglial research related to Alzheimer’s disease include the identification of biomarkers linked to abnormal synaptic pruning and inflammation. Studies from labs like Beth Stevens’ have uncovered how microglial dysfunction can lead to neurodegeneration. This foundational research is paving the way for developing innovative treatments aimed at altering microglial behavior to slow or prevent disease progression.
How does curiosity-driven research impact the understanding of microglial cells in brain health?
Curiosity-driven research plays a significant role in advancing our understanding of microglial cells and their impact on brain health. By exploring fundamental questions about microglial function, researchers can uncover unexpected links to diseases such as Alzheimer’s. These discoveries not only enhance scientific knowledge but also inform practical applications, leading to novel therapeutic strategies for improving mental health outcomes.
What funding sources support microglial research related to neurodegenerative diseases?
Microglial research related to neurodegenerative diseases is often supported by federal funding sources such as the National Institutes of Health (NIH). This funding is crucial for foundational studies that explore the roles of microglial cells in conditions like Alzheimer’s disease, enabling scientists to investigate complex mechanisms that lead to neurodegeneration and ultimately contribute to the development of new therapeutic approaches.
| Key Point | Details |
|---|---|
| Microglial Cells Function | They act as the brain’s immune system, patrolling for illness or injury. |
| Impact on Alzheimer’s and Other Disorders | Abnormal pruning by microglia is linked to Alzheimer’s, Huntington’s, and more. |
| Research Funding | Research supported primarily by NIH and federal grants, enabling significant discoveries. |
| Curiosity-Driven Innovation | Studies on microglia lead to breakthroughs in understanding disease mechanisms and treatment development. |
| Recognition and Impact | Beth Stevens received the MacArthur Fellowship for her pioneering work in this field. |
Summary
Microglial research is essential for understanding the brain’s immune system and its role in neurodegenerative diseases. The work led by scientists like Beth Stevens highlights the importance of foundational research in fostering breakthroughs that can ultimately lead to new treatments for diseases such as Alzheimer’s. Continued investment in microglial research promises to enhance our comprehension of these complex disorders and improve therapeutic strategies for millions affected.