Patient safety in medical research is a crucial aspect that demands unwavering attention, especially amid recent funding cuts that threaten oversight systems. The integrity of clinical trials is fundamentally tied to the ethical considerations guided by medical research ethics, ensuring that the rights and well-being of research participants are prioritized. With the halting of essential funding, the oversight typically provided by Institutional Review Boards (IRB) may become compromised, posing risks to the safety of individuals involved in studies. This disruption could lead to patients being subjected to poorly monitored research practices, which may undermine confidence in clinical trials altogether. Therefore, robust IRB oversight is not just a regulatory requirement; it is a vital component of maintaining trust and assurance in the human research landscape.
The protection of individuals in clinical studies is essential for the integrity of scientific exploration. In this context, research participant safety encompasses the ethical frameworks that govern medical studies, particularly when funding constraints may jeopardize critical oversight systems. Institutional Review Boards (IRBs) play an instrumental role in maintaining ethical standards by evaluating research methodologies and ensuring informed consent is obtained appropriately. However, when research funding is cut, the impact reverberates through the very systems designed to safeguard these human subjects, potentially leading to compromised studies. By addressing these ethical concerns within medical research, stakeholders can foster a safer environment for participants involved in transformative clinical trials.
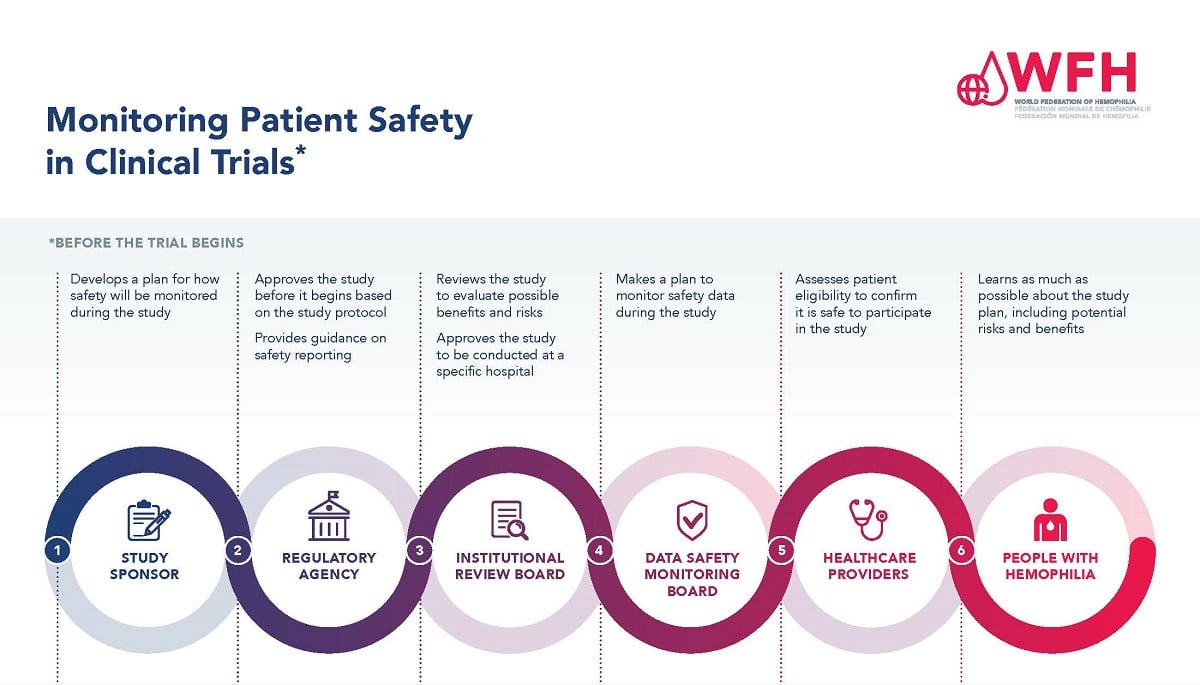
Understanding the Role of IRB Oversight in Patient Safety
Institutional Review Boards (IRBs) play a pivotal role in ensuring the rights and safety of participants involved in medical research. These boards are responsible for reviewing research proposals meticulously, assessing factors such as research design, ethical considerations, and potential risks to participants. In doing so, IRBs act as a safeguard, ensuring that study protocols adhere to ethical standards. Their oversight is particularly critical in clinical trials, where the safety of research participants can be at significant risk if not adequately monitored.
Moreover, IRBs facilitate training and support for investigators, helping them navigate the complex ethical landscape of medical research. They work closely with researchers to ensure informed consent is obtained appropriately and that participants are fully aware of their rights and any potential risks. This dedicated oversight ensures that studies are conducted ethically and transparently, reinforcing public trust in the research process.
Impact of Funding Cuts on Research Ethics and Patient Safety
The recent halt in federal funding has raised significant concerns regarding the ethical conduct of medical research, particularly in relation to the safety of participants. Cuts exceeding $2 billion have led to disruptions in critical processes managed by systems like SMART IRB, which ensure comprehensive oversight across multiple research sites. These cuts jeopardize ongoing clinical trials and the ability to implement necessary safety measures, ultimately putting the well-being of research participants at risk.
As funding diminishes, IRB functions may also be compromised, leading to a backlog of review processes and potential delays in implementing new studies. This can result in prolonged exposure to risks for participants who have already enrolled in research studies. The need for solid financial backing is essential; without it, the structural integrity of research ethics and participant safety could be undermined, risking a return to scenarios of mistrust previously experienced in medical research history.
Furthermore, funding cuts can also slow the progress of research innovations crucial for tackling pressing health issues, such as Alzheimer’s disease or cancer treatments. When research initiatives are put on hold due to financial constraints, it not only affects the individual studies but can also impact the larger scientific community’s ability to collaborate effectively, further jeopardizing advancements in healthcare.
Navigating Ethics in Multisite Research and IRB Collaboration
The rise in multisite research collaborations has necessitated the evolution of IRB oversight protocols, such as the introduction of single IRB (sIRB) models mandated by NIH policies. These models are designed to streamline the review process while ensuring consistent ethical standards across different institutions. The collaboration fosters efficient resource allocation and reduces redundancy in IRB reviews, ultimately promoting patient safety in medical research by ensuring that protections remain uniform regardless of the study’s location.
However, the challenges posed by funding cuts threaten to complicate these collaborative efforts significantly. Without adequate financial support, institutions may struggle to engage effectively with their IRB counterparts, leading to delays in study approvals and participant recruitment. This situation highlights the critical need for a reliable funding model that supports not only the research studies themselves but also the essential regulatory frameworks that uphold the integrity of the research process.
Challenges in Patient Recruitment Amidst Financial Constraints
Patient recruitment is a vital component of successful medical research, yet ongoing funding issues complicate this process. The rise in budget constraints can deter potential participants, who may view ongoing trials as less trustworthy or less likely to be properly regulated. The potential loss of confidence can have lasting effects, leading to decreased enrollment numbers and the risk of incomplete data when trials continue without adequate participant representation.
Moreover, as institutions rely on the goodwill and trust of their communities, the suspension of funding exacerbates skepticism toward the motivations behind clinical research endeavors. It is essential that researchers and institutions actively work to maintain transparency and address any concerns among potential participants. Building back this trust is a critical priority that requires not only ethical research practices but also consistent financial backing to uphold participant safety and rights.
The Future of Medical Research amid Funding Uncertainty
The landscape of medical research is evolving rapidly, and with financial uncertainties looming over federal funding, the path ahead seems fraught with challenges. Researchers face the dilemma of advancing innovative health solutions while navigating the complexities of disrupted funding streams that could hinder their ability to conduct ethical and comprehensive studies. Without reliable funding, the scope of research can become limited, impacting the technologies and treatments developed in the foreseeable future.
To navigate these waters successfully, institutions must advocate for the importance of funding not only for research projects but also for the infrastructure that supports patient safety in medical research. By fostering advocacy efforts and showcasing the benefits of investment in research ethics, institutions may work to stabilize funding sources and prioritize the protection of research participants, ensuring the integrity and effectiveness of medical innovation moving forward.
Upholding Informed Consent Standards in Research
Informed consent is a foundational pillar of ethical medical research, ensuring that participants understand the implications of their involvement in studies. The role of IRBs in reviewing consent documents and processes is critical to this end. However, with funding cuts, the ability of institutions to provide adequate training and resources to researchers may be compromised. This could lead to lapses in the informed consent process, jeopardizing the understanding and rights of participants.
The necessity of clear communication and guidance for both researchers and participants has never been more crucial. Institutions must recognize the potential impacts of decreased funding on these processes and strive to maintain rigorous training programs. Protecting informed consent standards safeguards participant autonomy and trust, essential components for the success of any research study.
Historical Context of Ethical Oversight in Medical Research
Understanding the history of medical research ethics is fundamental to grasping the importance of current oversight mechanisms like IRBs. Historical events, such as the Tuskegee Study and the experiments conducted during World War II, underline the dire need for ethical scrutiny and protection of research participants. These egregious violations resulted in significant reforms aimed at preventing future abuses in human research, laying the groundwork for contemporary ethical standards.
The tragic lessons from the past serve as a constant reminder of the importance of IRB oversight today. Continued education around these historical contexts can reinforce the commitment to ethical practices, prompting institutions to prioritize participant safety actively. This historical perspective can unify researchers, patients, and the broader community in their shared goals of responsible research conduct.
Strengthening Community Engagement in Research Practices
Effective community engagement plays a significant role in enhancing the ethical conduct of medical research. By establishing trust between researchers and the communities they serve, institutions can foster an environment that facilitates ethical participation and supports informed consent processes. Robust dialogue between researchers and community representatives can address potential concerns and emphasize the importance of participant safety, ultimately enriching the research landscape.
As funding cuts threaten the viability of outreach programs essential for community engagement, researchers must navigate these challenges proactively. By finding innovative ways to maintain community partnerships and leveraging existing relationships, researchers can ensure that ethical research practices continue to flourish, prioritizing participant safety and reinforcing the public’s trust in medical research.
The Importance of Training and Education in Research Ethics
Training for researchers is critical in maintaining ethical standards throughout the medical research process. Institutions must invest in comprehensive educational programs that emphasize the importance of participant safety and the ethical responsibilities associated with conducting research. By equipping researchers with the knowledge and skills necessary to navigate complex ethical dilemmas, the integrity of research can be upheld even in the face of funding cuts.
Furthermore, ongoing education and awareness initiatives can promote a culture of ethics within research institutions. Encouraging dialogue around the historical context of research ethics and the key role of IRBs in safeguarding participant rights can empower researchers to prioritize these values actively. Investing in training not only enhances individual awareness but also elevates the overall ethical standards of medical research, contributing to the long-term protection of participants.
Frequently Asked Questions
What is the role of IRB oversight in ensuring patient safety in medical research?
Institutional Review Boards (IRBs) play a crucial role in protecting patient safety in medical research by reviewing research proposals to ensure ethical standards are met. They assess study designs, informed consent processes, and potential risks to participants, helping mitigate harm during clinical trials and maintaining participant welfare.
How do funding cuts affect the safety of patients in medical research?
Funding cuts significantly impact the safety of patients in medical research by halting ongoing studies and limiting the ability of IRBs to operate effectively. This can lead to delays in clinical trials, reduced oversight, and increased risks for research participants, ultimately compromising their safety and trust in the research process.
What is the importance of medical research ethics in protecting patient safety during clinical trials?
Medical research ethics are vital in protecting patient safety during clinical trials, as they establish the framework for IRB oversight and the conduct of research. Upholding ethical principles ensures that patients’ rights are respected, risks are minimized, and informed consent is properly obtained, thus safeguarding the well-being of research participants.
What measures do IRBs take to ensure the safety of research participants in medical studies?
IRBs implement various measures to ensure the safety of research participants, including rigorous review of research proposals, continuous monitoring of study risks, and ensuring informed consent processes are transparent. They serve as a critical safety net, ensuring that ethical standards are maintained throughout clinical trials.
How do historical events shape the current practices in patient safety for medical research?
Historical events, such as unethical medical experiments, have led to the establishment of strict regulations and oversight mechanisms to ensure patient safety in medical research. These incidents underscore the importance of informed consent and IRB oversight, shaping current practices to protect the rights and welfare of research participants.
What role does collaboration play in enhancing patient safety in multi-site clinical trials?
Collaboration among hospitals, universities, and research institutions enhances patient safety in multi-site clinical trials by streamlining the IRB oversight process through systems like SMART IRB. This unified approach minimizes administrative burdens, facilitates quicker approvals, and enhances the overall oversight of research studies, ultimately benefitting patient safety.
Why is informed consent critical for patient safety in medical research studies?
Informed consent is critical for patient safety in medical research studies as it ensures that participants are fully aware of the study’s risks, benefits, and procedures before agreeing to participate. This process empowers patients to make educated choices about their involvement in research, reinforcing their safety and autonomy.
What are the potential consequences of the halt in federal funding for patient safety in ongoing medical research?
The halt in federal funding can lead to the suspension of ongoing medical research, jeopardizing the safety of participants already involved in studies. This disruption can limit the resources available for IRBs and research professionals, undermining oversight and increasing risks to participants’ health and safety.
How does patient safety in medical research relate to public trust in clinical trials?
Patient safety in medical research is intrinsically linked to public trust in clinical trials. Ensuring robust IRB oversight, ethical practices, and transparency fosters confidence among potential research participants and the public, essential for the successful conduct of medical studies and advancements in healthcare.
What resources do IRBs provide to enhance the safety of research participants?
IRBs provide various resources to enhance the safety of research participants, including training for researchers on ethical guidelines, support in the informed consent process, and ongoing monitoring of trials. These resources help ensure that participant safety remains a top priority throughout the research lifecycle.
| Key Point | Details |
|---|---|
| Funding Cuts Impact | The Trump administration’s freeze of over $2 billion in federal research grants to Harvard has disrupted patient safety efforts. |
| Role of SMART IRB | SMART IRB facilitates oversight of studies across multiple sites, ensuring proper regulations are followed for patient safety. |
| Importance of IRBs | IRBs protect patients by reviewing proposals, ensuring informed consent, and assessing research risks and benefits. |
| Historical Context | Past unethical practices in medical research have led to the need for careful oversight to protect participants’ rights. |
| Current Issues | Funding cuts lead to halted studies and increased risks for participants, undermining public trust in research. |
| Future Implications | Ongoing cuts to research funding threaten patient safety and the integrity of medical research efforts. |
Summary
Patient safety in medical research is critically jeopardized by funding cuts that halt crucial oversight systems. The recent freeze on federal grants has significantly disrupted efforts to maintain the rights and safety of participants in medical studies. Without adequate funding and support, essential regulatory bodies like the SMART IRB are unable to function effectively, putting participants at risk and undermining public trust in the research process. It is imperative that we sustain funding for medical research to ensure the ethical treatment and safety of all involved.